Rechargeable batteries based on magnesium, rather than lithium, have the potential to extend electric vehicle range by packing more energy into smaller batteries. But unforeseen chemical roadblocks have slowed scientific progress.
Rechargeable batteries based on magnesium, rather than lithium, have the potential to extend electric vehicle range by packing more energy into smaller batteries. But unforeseen chemical roadblocks have slowed scientific progress.
And the places where solid meets liquid – where the oppositely charged battery electrodes interact with the surrounding chemical mixture known as the electrolyte – are the known problem spots.
Now, a research team at the U.S. Department of Energy’s Joint Center for Energy Storage Research, led by scientists at Lawrence Berkeley National Laboratory (Berkeley Lab), has discovered a surprising set of chemical reactions involving magnesium that degrade battery performance even before the battery can be charged up.
Read more at DOE / Lawrence Berkeley National Laboratory
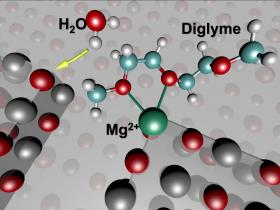
Image: These molecular models show the initial state of battery chemistry that leads to instability in a test cell featuring a magnesium (Mg) anode. (Credit: Berkeley Lab)