For some crystalline catalysts, what you see on the surface is not always what you get in the bulk, according to two studies led by the Department of Energy’s Oak Ridge National Laboratory.
For some crystalline catalysts, what you see on the surface is not always what you get in the bulk, according to two studies led by the Department of Energy’s Oak Ridge National Laboratory.
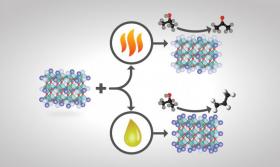
The investigators discovered that treating a complex oxide crystal with either heat or chemicals caused different atoms to segregate on the surface, i.e., surface reconstruction. Those differences created catalysts with dissimilar behaviors, which encouraged different reaction pathways and ultimately yielded distinct products.
By using thermal and chemical treatments, catalyst designers may be able to drive industrially important chemical reactions to improve yields of desired products and reduce unwanted products so post-reaction separation costs can be significantly lowered.
“The surface of a catalyst is a playground for the molecules to do the chemical reaction,” said ORNL chemist Zili Wu, the senior author of two recent papers about the effect of the atomic composition of a catalyst surface on acid-base chemistry. “If you can tune your catalyst to obtain the desired product, i.e., achieve high selectivity, you will reduce the side products. Then you don’t need costly and energy-intensive downstream chemical separation as much.”
Read more at DOE/Oak Ridge National Laboratory
Image: How perovskite catalysts are made and treated changes their surface compositions and ultimate product yields. If certain perovskite catalysts of the formula ABO3 are heat-treated, the catalyst's surface terminates predominantly with A (a rare-earth metal cation depicted in light purple) and less with B (a transition-metal cation shown in dark purple)--and isopropanol conversion over this basic catalyst primarily yields acetone. If the same catalyst is treated chemically instead of with heat, the catalyst's surface termination is instead mostly B and less A and is more acidic--and isopropanol conversion yields mainly propylene. (Credit: Image credit: Oak Ridge National Laboratory, U.S. Dept. of Energy; illustrator Adam Malin)