Methane gas, a vast natural resource, is often disposed of through burning, but new research by scientists at MIT could make it easier to capture this gas for use as fuel or a chemical feedstock.
Methane gas, a vast natural resource, is often disposed of through burning, but new research by scientists at MIT could make it easier to capture this gas for use as fuel or a chemical feedstock.
Many oil wells burn off methane — the largest component of natural gas — in a process called flaring, which currently wastes 150 billion cubic meters of the gas each year and generates a staggering 400 million tons of carbon dioxide, making this process a significant contributor to global warming. Letting the gas escape unburned would lead to even greater environmental harm, however, because methane is an even more potent greenhouse gas than carbon dioxide is.
Why is all this methane being wasted, when at the same time natural gas is touted as an important “bridge” fuel as the world steers away from fossil fuels, and is the centerpiece of the so-called shale-gas revolution? The answer, as the saying goes in the real estate business, is simple: location, location, location.
The wells where methane is flared away are primarily being exploited for their petroleum; the methane is simply a byproduct. In places where it is convenient to do so, methane is captured and used to generate electrical power or produce chemicals. However, special equipment is needed to cool and pressurize methane gas, and special pressurized containers or pipelines are needed to transport it. In many places, such as offshore oil platforms or remote oil fields far from the needed infrastructure, that’s just not economically viable.
Read more at Massachusetts Institute of Technology
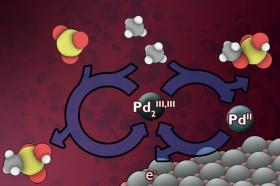
Image: MIT chemistry professor Yogesh Surendranath and three colleagues have found a way to use electricity, which could potentially come from renewable sources, to convert methane into derivatives of methanol. The researchers developed a low-temperature electrochemical process that would continuously replenish a catalyst material that can rapidly carry out the conversion. (Credit: Courtesy of the researchers MIT)