Modern batteries power everything from cars to cell phones, but they are far from perfect – they catch fire, they perform poorly in cold weather and they have relatively short lifecycles, among other issues. Now researchers from the University of Houston have described a new class of material that addresses many of those concerns in Nature Materials.
Modern batteries power everything from cars to cell phones, but they are far from perfect – they catch fire, they perform poorly in cold weather and they have relatively short lifecycles, among other issues. Now researchers from the University of Houston have described a new class of material that addresses many of those concerns in Nature Materials.
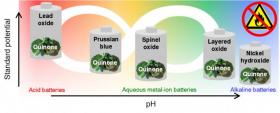
The researchers, led by Yan Yao, associate professor of electrical and computer engineering, report their use of quinones – an inexpensive, earth-abundant and easily recyclable material – to create stable anode composites for any aqueous rechargeable battery.
“This new material is cheap and chemically stable in such a corrosive environment,” said Yao, who is also a principal investigator with the Texas Center for Superconductivity at UH, with an appointment to the chemical and biomolecular engineering faculty. The material also can be used to create a “drop-in replacement” for current battery anodes, allowing the new material to be used without changing existing battery manufacturing lines, he said.
“This can get to market much faster,” he said.
Read more at University of Houston
Image: UH researchers have discovered a new material that has proven an effective anode for acid and alkaline batteries, including emerging aqueous metal-ion batteries, offering the promise of safe, long-lasting batteries that work across a range of temperatures. (Credit: University of Houston)