Engineers at the University of California, Riverside have developed a new way to recover almost 100 percent of the water from highly concentrated salt solutions. The system will alleviate water shortages in arid regions and reduce concerns surrounding high salinity brine disposal, such as hydraulic fracturing waste.
Engineers at the University of California, Riverside have developed a new way to recover almost 100 percent of the water from highly concentrated salt solutions. The system will alleviate water shortages in arid regions and reduce concerns surrounding high salinity brine disposal, such as hydraulic fracturing waste.
The research, which involves the development of a carbon nanotube-based heating element that will vastly improve the recovery of fresh water during membrane distillation processes, was published today in the journal Nature Nanotechnology. David Jassby, an assistant professor of chemical and environmental engineering in UCR’s Bourns College of Engineering, led the project.
While reverse osmosis is the most common method of removing salt from seawater, wastewater, and brackish water, it is not capable of treating highly concentrated salt solutions. Such solutions, called brines, are generated in massive amounts during reverse osmosis (as waste products) and hydraulic fracturing (as produced water), and must be disposed of properly to avoid environmental damage. In the case of hydraulic fracturing, produced water is often disposed of underground in injection wells, but some studies suggest this practice may result in an increase in local earthquakes.
One way to treat brine is membrane distillation, a thermal desalination technology in which heat drives water vapor across a membrane, allowing further water recovery while the salt stays behind. However, hot brines are highly corrosive, making the heat exchangers and other system elements expensive in traditional membrane distillation systems. Furthermore, because the process relies on the heat capacity of water, single pass recoveries are quite low (less than 10 percent), leading to complicated heat management requirements.
Read more at University of California, Riverside
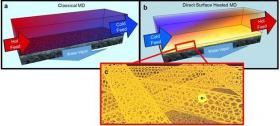
Image: Hot brines used in traditional membrane distillation systems are highly corrosive, making the heat exchangers and other system elements expensive, and limiting water recovery (a). To improve this, UCR researchers developed a self-heating carbon nanotube-based membrane that only heats brine at the membrane surface (b), where the porous carbon nanotube layer acts as a Joule heater (c). (Credit: UC Riverside)