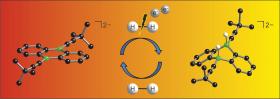
Hydrogen (H2) is an extremely simple molecule and yet a valuable raw material which as a result of the development of sophisticated catalysts is becoming more and more important. In industry and commerce, applications range from food and fertilizer manufacture to crude oil cracking to utilization as an energy source in fuel cells. A challenge lies in splitting the strong H-H bond under mild conditions. Chemists at Goethe University have now developed a new catalyst for the activation of hydrogen by introducing boron atoms into a common organic molecule. The process, which was described in theAngewandte Chemie journal, requires only an electron source in addition and should therefore be usable on a broad scale in future.
Hydrogen (H2) is an extremely simple molecule and yet a valuable raw material which as a result of the development of sophisticated catalysts is becoming more and more important. In industry and commerce, applications range from food and fertilizer manufacture to crude oil cracking to utilization as an energy source in fuel cells. A challenge lies in splitting the strong H-H bond under mild conditions. Chemists at Goethe University have now developed a new catalyst for the activation of hydrogen by introducing boron atoms into a common organic molecule. The process, which was described in theAngewandte Chemie journal, requires only an electron source in addition and should therefore be usable on a broad scale in future.
The high-energy content of the hydrogen molecule meets with a particularly stable bonding situation. It was Paul Sabatier who in 1897 detected for the first time that metals are suitable catalysts for splitting the molecule and harnessing elementary hydrogen for chemical reactions. In 1912 he was awarded the Nobel Prize for Chemistry for this important discovery. The hydrogenation catalysts mostly used today contain toxic or expensive heavy metals, such as nickel, palladium or platinum. Only ten years ago non-metal systems based on boron- and phosphorous compounds were discovered which allow comparable reactions.
Continue reading at Goethe University Frankfurt
Photo via Goethe University Frankfurt