At first glance, magnetite appears to be a rather inconspicuous grey mineral. But on an atomic scale, it has remarkable properties: on magnetite, single metal atoms are held in place, or they can be made to move across the surface. Sometimes several metal atoms on magnetite form small clusters. Such phenomena can dramatically change the chemical activity of the material. Atomic processes on the magnetite surface determine how well certain metal atoms can serve as catalysts for chemical reactions.
Scientists at TU Wien (Vienna), together with colleagues from Utrecht University, can now watch single platinum atoms form tiny clusters. Carbon monoxide plays a dual role in this process: It allows single platinum atoms to move and form pairs, and then it holds these pairs together for a long time. Only by increasing the temperature can the pair-bonds between platinum atoms can be broken.
At first glance, magnetite appears to be a rather inconspicuous grey mineral. But on an atomic scale, it has remarkable properties: on magnetite, single metal atoms are held in place, or they can be made to move across the surface. Sometimes several metal atoms on magnetite form small clusters. Such phenomena can dramatically change the chemical activity of the material. Atomic processes on the magnetite surface determine how well certain metal atoms can serve as catalysts for chemical reactions.
Scientists at TU Wien (Vienna), together with colleagues from Utrecht University, can now watch single platinum atoms form tiny clusters. Carbon monoxide plays a dual role in this process: It allows single platinum atoms to move and form pairs, and then it holds these pairs together for a long time. Only by increasing the temperature can the pair-bonds between platinum atoms can be broken.
Lonely Atoms
It sounds a bit like an unhappy love story: "Two platinum atoms would actually like to be together, but the magnetite surface keeps them apart", says Roland Bliem (TU Wien). Together with Professor Gareth Parkinson, Professor Ulrike Diebold and their colleagues, he analysed the behaviour of platinum atoms using a scanning tunnelling microscope.
"When a platinum atom hits the magnetite surface, it is kept in place by the oxygen atoms in the magnetite. The atoms always end up alone. On other surfaces, pair formation would be favoured, but magnetite does not allow that", says Roland Bliem. The platinum atoms sit on specific places on the magnetite crystal and cannot get away without outside help.
Continue reading at EurekAlert!
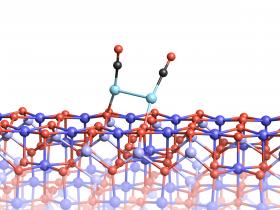
Image: Two platinum atoms on the magnetite surface can bond, if they are attached to CO molecules.
Credits: Vienna University of Technology