Our society is in need of ammonia more than ever.
Chemical fertilizers, plastic, fibers, pharmaceuticals, refrigerants in heat pumps, and even explosives all use ammonia as raw material. Moreover, ammonia has been suggested as a hydrogen carrier recently because of its high hydrogen content.
Our society is in need of ammonia more than ever.
Chemical fertilizers, plastic, fibers, pharmaceuticals, refrigerants in heat pumps, and even explosives all use ammonia as raw material. Moreover, ammonia has been suggested as a hydrogen carrier recently because of its high hydrogen content.
In the Haber-Bosch process, which is the main method of ammonia synthesis, nitrogen reacts with hydrogen using a metal catalyst to produce ammonia. However, this industrial process is conducted at 200 atm and high reaction temperatures of nearly 500°C. Additionally, ammonia production requires using much natural gas, so scientists have been looking for alternative methods to sustainably synthesize ammonia at low temperature.
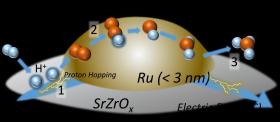
In a recent study, researchers from Waseda University and Nippon Shokubai Co. Ltd. achieved a highly efficient ammonia synthesis at low temperature, with the highest yield ever reported.
Read more at Waseda University
Image: Proton hopping plays an important role in the reaction, as it activates nitrogen gas even at low temperatures and moderates the harsh condition requirements. (Credit: Waseda University)